
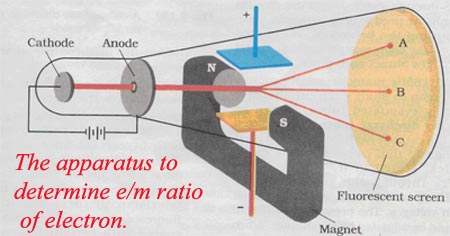
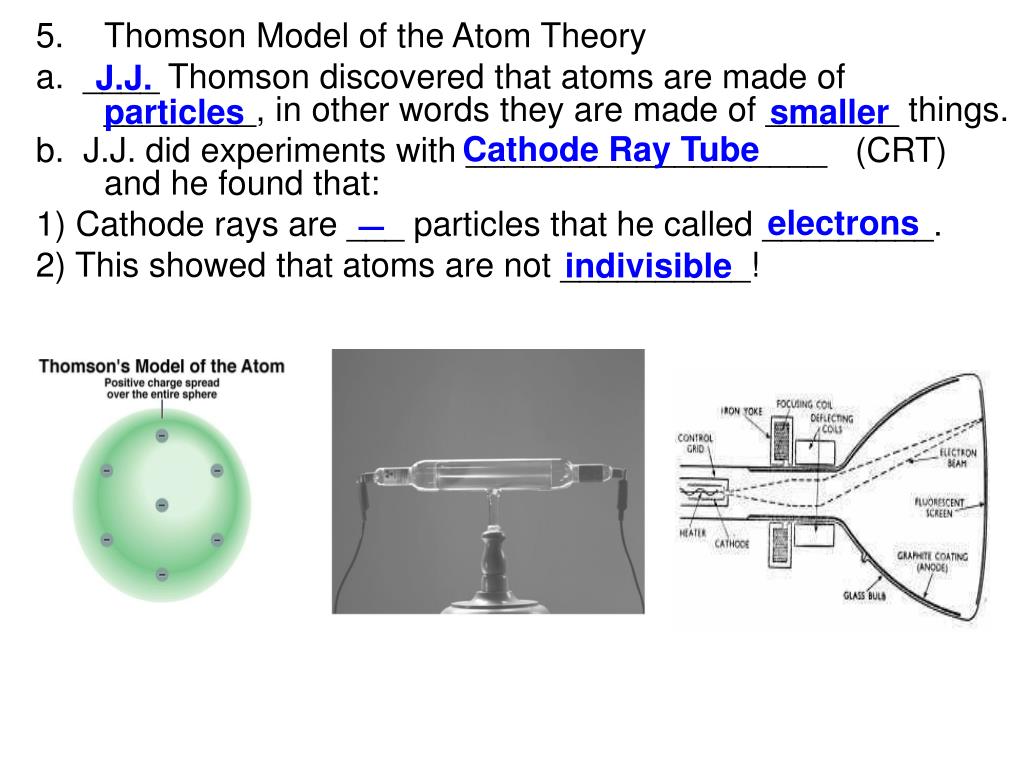
Subsequently, Thomson tried to validate the properties of these particles: basically the electric charge and the reaction between them. In his experiments, Thomson determined the deflection of the ray in its path from the cathode to the anode. By painting the anode of the tube with a material such as phosphorus or lead, a reaction is generated at the positive end just when the particle beam hits it. There is the origin of the name of this type of tools, since they are called cathode rays due to the point of exit of the particles inside the tube. Cathode Raysīeams of electrons are directed from the cathode to the anode. By this configuration the circulation of a particle beam from the cathode to the anode of the tube is induced. They are also sealed on both sides and are subjected to high voltage levels by electrification of two electrodes placed on the cathode of the device. These tubes are electrified with a battery that polarizes the tube to have a negative charge end (cathode) and a positively charged end (anode). Cathode ray tubes are glass tubes whose air content has been emptied almost entirely. Thomson conducted several tests with cathode ray tubes to test the properties of subatomic particles and lay the groundwork for his model. The Thomson model was popularly known in England as a plum pudding model, since the electron distribution proposed by Thomson was similar to the disposition of the plums in that dessert. If the electrons moved through the positive"mass", the internal forces within the electrons were balanced by the positive charge that was automatically generated around the orbit. The electrons had stable orbits, argument based on the Gaussian Law. The electrons could derive freely inside the atom. In the words of Thomson:"negatively electrified corpuscles"- that is, electrons - are contained within the uniform mass of positive charge. This positive charge is evenly distributed in the atom. There is a source of positive charge that neutralizes the negative charge of the electrons. Therefore, the proposal consisted of a dispersed configuration of all the negative charges within the atomic structure which, in turn, was made up of a uniform mass of positive charge. It is worth noting that Thomson's atomic model was proposed shortly after the discovery of the electron but before knowing the existence of an atomic nucleus. In addition, Thomson was responsible for the discovery of the electron at the end of the 19th century. I was surprised at this - it meant that these particles must be extremely small. "Could anything at first sight seem more impractical than a body which is so small that its mass is an insignificant fraction of the mass of an atom of hydrogen?" I was convinced that these particles were the basis of matter, including atoms.This atomic model was proposed by the English scientist Joseph John"J.J."Thomson in 1904, with the purpose of explaining the composition of atoms based on the notions of which we had knowledge by then. I calculated the charge-to-mass ratio of one of these particles to be 1.758820150(44)×10^11 C/kg, which means that there is a huge negative charge on a extremely small particle. To do this, I bent the ray with a magnet and measured how much they were bent, and also measured how much energy the ray carried. In my last experiment, I found out the ratio of the mass of a particle to its charge. I couldn't know for sure what these particles (I called them corpuscles) actually were until I experimented again. My conclusion was that cathode cathode rays are negative charges carried by material particles. My contemporaries had already experimented with their ideas.Ī: Incredible! So are the ray and its charge the same thing?

It also made sense to me that the rays were made up of material particles, which I thought were all made up of ether anyway. It made sense that these rays were similar to normal light waves, which they thought traveled in "ether", an invisible fluid. Many of my contemporaries have tried to explain this mysterious glow. When we pump out the air and put a high voltage across it, a curious fluorescent light emits from it. Cathode ray tubes are simple glass tubes with wires at opposite ends. Back in 1897, I was experimenting with cathode rays at Cambridge University. I've managed to shed some light about how the universe works.Ī: Great! Why don't you tell us more about the electrons? T: Well, through my experimenting, I discovered the electron and developed a new model of the atom. Would you mind telling us what they are exactly? I'm happy to answer any questions you might have about my work.Ī: Your recent discoveries about the depths of the atom have made big headlines.
Thomson: Why thank you, it's a pleasure to meet you too.


 0 kommentar(er)
0 kommentar(er)
